Custom organic synthesis, analytical service, active pharmaceutical ingredient (API) synthesis and production
The demand for custom-made molecules and label claims control is growing, so we’re always growing our offering to stay ahead.
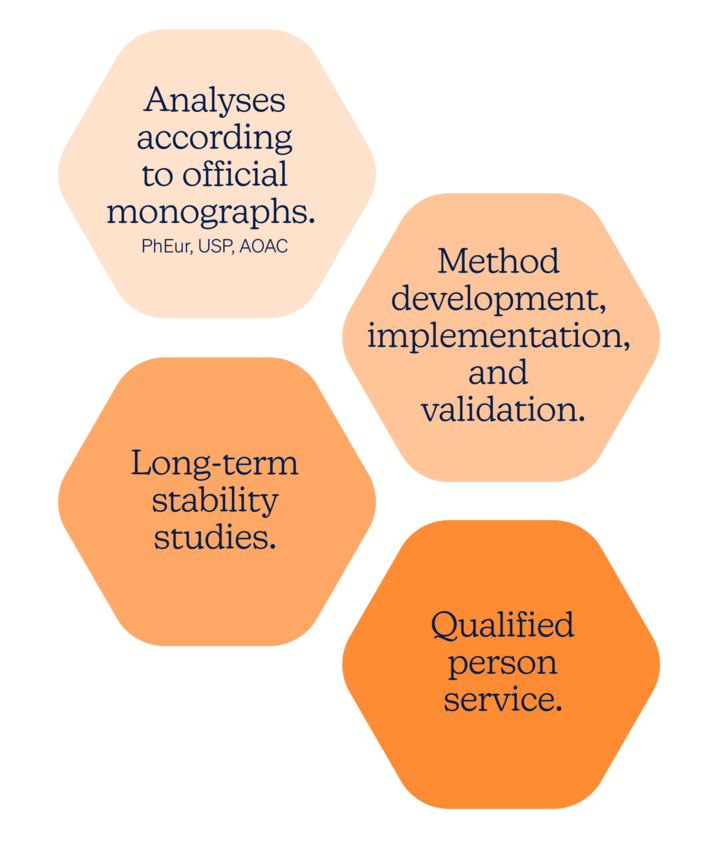
That’s why we offer our partners a completely integrated range of organic chemistry and analytical services, cGMP certified labs and unmatched knowledge of vitamin K2.